Ammonia Oxidation Solutions
By providing highly selective and efficient ammonia oxidation solutions, we support a broad variety of final applications that require nitric oxide as precursor: nitrates for fertilizers to feed the world, nitrates for explosives to gain steel for building, hydroxylamines and methacrylates for any kind of plastics, or caprolactarme for clothing and fashion.
Find out more about:
Ostwald Process
Manufacturing of Nitric Acid HNO3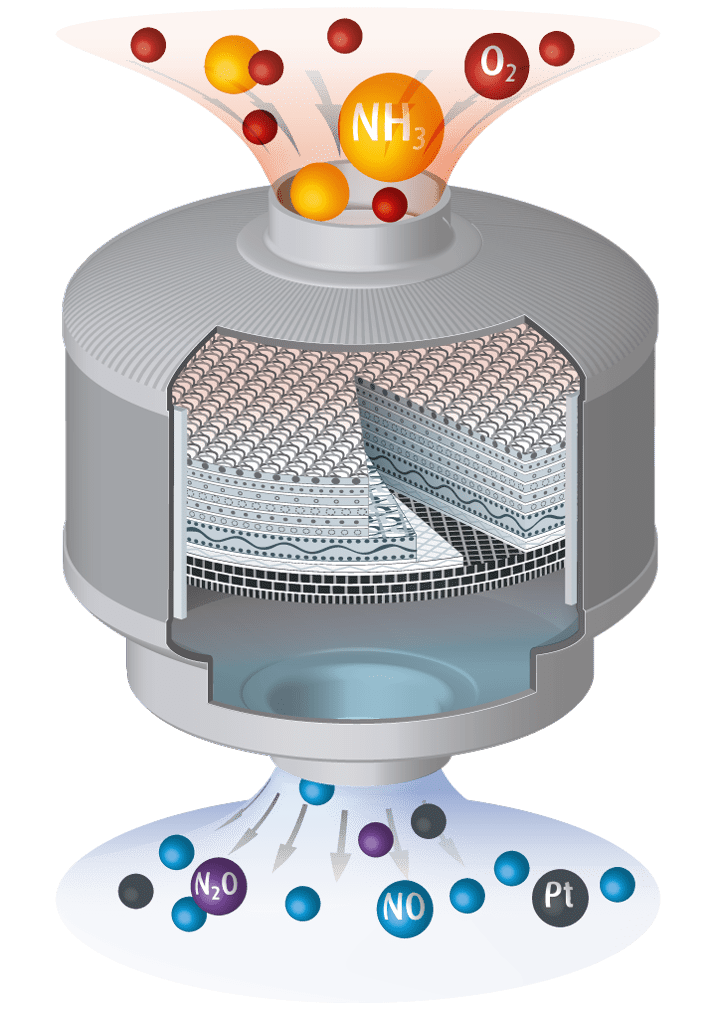
Step 1: Catalytic oxidation of ammonia:
- Synthesis of NO by means of PtRh catalyst gauzes:
4 NH3 + 5 O2 ⇒ 4 NO + 6 H2O - Formation of N2O is a side reaction that can’t be completely avoided:
4 NH3 + 4 O2 ⇒ 2 N2O + 6 H2O - Temperatures of about 800 to 950°C
- Pressure in the range between 1 and 15 bar
- Contact time of gas mix with catalyst in the range of 1/1.000 sec
Step 2: Generation of Nitrogendioxide and dimerisation
-
2 NO + O2 ⇒ 2 NO2 ⇒ N2O4
Step 3: Oxidation and absorption in column to yield final product
-
2 N2O4 + O2 + 2 H2O ⇒ 4 HNO3